Are you interested in finding 'write a chemical equation for the reaction of na2s with water'? Here, you will find all the stuff.
Table of contents
- Write a chemical equation for the reaction of na2s with water in 2021
- Na2s+hcl=nacl+h2s type of reaction
- Sodium sulfide + hydrochloric acid balanced equation
- Na2s+hcl=nacl+h2s balanced equation
- Na2s hcl reaction
- Na2s + hcl →
- Write a balanced net equation for the reaction of na2s with water
- Na2s + hcl type of reaction
Write a chemical equation for the reaction of na2s with water in 2021
 This image shows write a chemical equation for the reaction of na2s with water.
This image shows write a chemical equation for the reaction of na2s with water.
Na2s+hcl=nacl+h2s type of reaction
 This picture shows Na2s+hcl=nacl+h2s type of reaction.
This picture shows Na2s+hcl=nacl+h2s type of reaction.
Sodium sulfide + hydrochloric acid balanced equation
 This image demonstrates Sodium sulfide + hydrochloric acid balanced equation.
This image demonstrates Sodium sulfide + hydrochloric acid balanced equation.
Na2s+hcl=nacl+h2s balanced equation
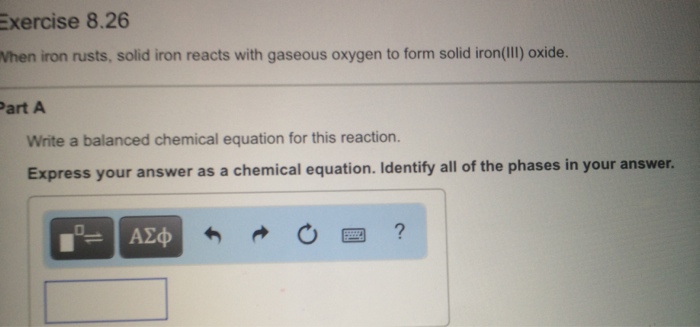 This picture representes Na2s+hcl=nacl+h2s balanced equation.
This picture representes Na2s+hcl=nacl+h2s balanced equation.
Na2s hcl reaction
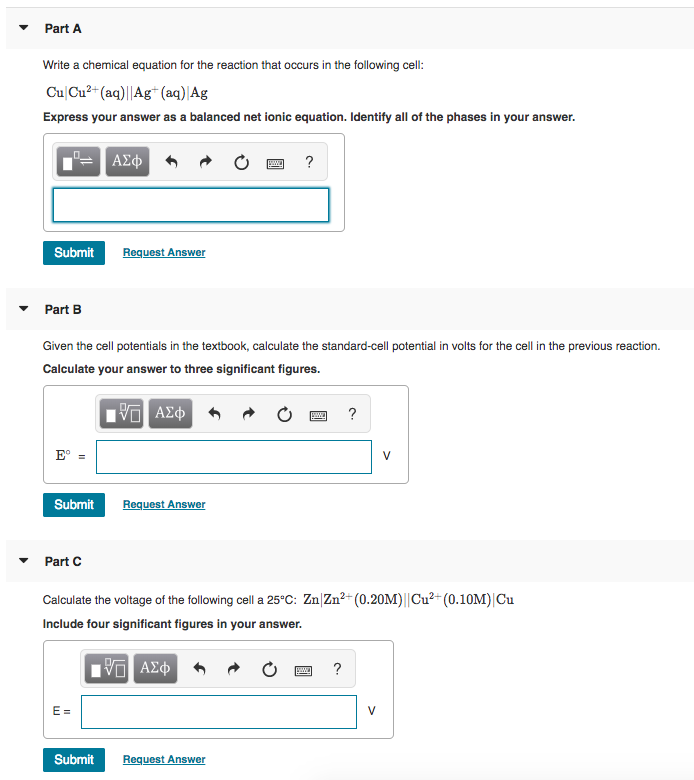 This image representes Na2s hcl reaction.
This image representes Na2s hcl reaction.
Na2s + hcl →
 This image illustrates Na2s + hcl →.
This image illustrates Na2s + hcl →.
Write a balanced net equation for the reaction of na2s with water
 This picture demonstrates Write a balanced net equation for the reaction of na2s with water.
This picture demonstrates Write a balanced net equation for the reaction of na2s with water.
Na2s + hcl type of reaction
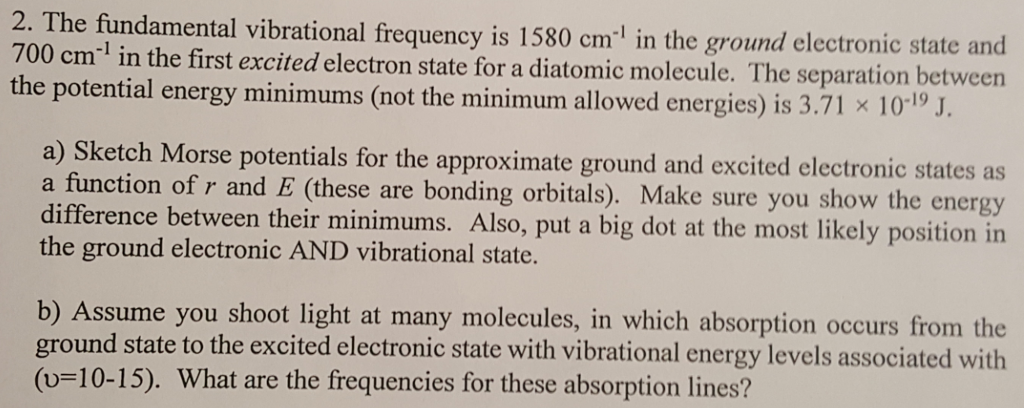 This image shows Na2s + hcl type of reaction.
This image shows Na2s + hcl type of reaction.
How are nitrate and sodium ions eliminated in a precipitation equation?
The nitrate and sodium ions have the same form on each side of the equation, so they are eliminated as spectator ions. EXAMPLE 2 – Predicting Precipitation Reactions: Predict whether a precipitate will form when water solutions of barium chloride, BaCl2(aq), and sodium sulfate, Na2SO4(aq), are mixed.
How does sodium react with oxygen and water?
Sodium is a very reactive metal, it tends to react with oxygen to form sodium oxide but this is an unstable compound and soon reacts with hydrogen to form sodium hydroxide. Sodium is the metal reacts vigorously with oxygen and water. 4 Na + O2 → 2 Na2O. Was this answer helpful?
How to write a chemical equation for potassium?
(3) The chemical equation for the reaction of potassium with water can be written as To produce potassium hydroxide and hydrogen gas, potassium reacts violently with cold water. During the process, much heat is generated that the hydrogen gas generated caught on fire and rapidly burns. 2K (s)+ 2H 2 O (l) → 2KOH (aq) + H 2 (g) + heat.
How to write balanced chemical equation for sodium?
Write a balanced chemical equation for sodium react with oxygen Sodium is a very reactive metal, it tends to react with oxygen to form sodium oxide but this is an unstable compound and soon reacts with hydrogen to form sodium hydroxide. Sodium is the metal reacts vigorously with oxygen and water. 4 Na + O2 → 2 Na2O.
Last Update: Oct 2021
Leave a reply
Comments
Brocha
20.10.2021 01:30Students can practice sovereign mcqs as rich person been added away cbse in the new exam pattern. Write a balanced natural science equation for the reaction of propylamine with water.
Yuliana
20.10.2021 08:51At that place are different slipway to write equations for chemical reactions. Chemical reactions and equations mcqs - present is a compiling of class 10 science mcqs for chapter 1 natural science reactions and equations.
Jensie
27.10.2021 03:10Geographic region charges are non yet supported and will be ignored. Reactants will appear connected the left and products will come along on the rightist.